 Commonwealth Consolidated Acts
Commonwealth Consolidated Acts Commonwealth Consolidated Acts
Commonwealth Consolidated ActsWhen section applies to new brands
(1) Subject to subsections (2), (3), (3A) and (3B), this section applies to a brand (the new brand ) of a pharmaceutical item (the trigger item ) that is not a combination item if:
(a) a determination under subsection 85(6) comes into force in relation to the new brand of the trigger item on a day (the determination day ); and
(b) on the day before the determination day, the new brand of the trigger item was not a listed brand of the trigger item; and
(c) on the day before the determination day:
(i) a brand (the existing brand ) of a pharmaceutical item (the existing item ) was a listed brand of the existing item; and
(ii) the new brand of the trigger item is bioequivalent or biosimilar to the existing brand of the existing item; and
(iii) the trigger item and existing item have the same drug and manner of administration.
Note: For the purposes of paragraph (c), the new brand and the existing brand may be the same brand, or the trigger item and the existing item may be the same pharmaceutical item.
Circumstances in which section does not apply
(2) This section does not apply in relation to the new brand of the trigger item if:
(a) the trigger item is in a class of pharmaceutical items to which a 12.5%, 16% or 25% administrative price reduction has applied; or
(b) another pharmaceutical item that has the same drug and manner of administration as the new brand of the trigger item is in a class of pharmaceutical items to which a 12.5%, 16% or 25% administrative price reduction has applied; or
(c) if the drug that is in the trigger item is in a therapeutic group--a pharmaceutical item that:
(i) has another drug that is in that group; and
(ii) has the same manner of administration as the new brand of the trigger item;
is in a class of pharmaceutical items to which a 12.5%, 16% or 25% administrative price reduction has applied; or
(d) on the day before the determination day:
(i) the approved ex - manufacturer price of a listed brand of the existing item on 1 January 2016; or
(ii) if subparagraph (i) does not apply--the original approved ex - manufacturer price of the first listed brand of the existing item;
has, by virtue of previous price reductions, been reduced by 60% or more.
(2A) If the approved ex - manufacturer price mentioned in subparagraph (2)(d)(i) or (ii) is by reference to a different pricing quantity than the pricing quantity on the day before the determination day, the approved ex - manufacturer price mentioned in that subparagraph is taken to be the amount that the approved ex - manufacturer price would have been had the pricing quantity been the same as the pricing quantity on the day before the determination day.
(3) This section does not apply in relation to the new brand of the trigger item if:
(a) any of the following has applied:
(ia) a determination under paragraph (6A)(b);
(ib) subsection 99ACF(1) or (2) because of item 4A, 4B or 8 in the table in subsection 99ACF(1);
(ii) subsection 99ACF(1) or (2) because of repealed section 99ACH;
(iii) repealed subsection 99ACF(2AB) or (2AC);
(iv) section 99ACQ;
(v) subsection 99ACR(3) or (4);
in relation to:
(b) the new brand, or another listed brand, of the trigger item; or
(c) a listed brand of another pharmaceutical item that has the same drug and manner of administration as the new brand of the trigger item; or
(d) if the drug that is in the trigger item is in a therapeutic group--a listed brand of a pharmaceutical item that:
(i) has another drug that is in that group; and
(ii) has the same manner of administration as the new brand of the trigger item.
Note: For the purposes of subparagraph (a)(i), subsections (5) and (5A) of this section are taken not to have applied in relation to a brand of a pharmaceutical item in some cases: see section 99AEI and subsection (6B) of this section.
(3A) This section does not apply in relation to the new brand of the trigger item if:
(a) the new brand of the trigger item is a new presentation of an existing listed brand of a pharmaceutical item; and
(b) the determination day in relation to the new brand of the trigger item is on or before the fifth anniversary of the drug in the pharmaceutical item being on F1; and
(c) the responsible person for the new brand of the trigger item is the same person as the responsible person for the existing listed brand of the pharmaceutical item; and
(d) either of the following apply:
(i) there is not another brand of the pharmaceutical item that has the drug that is a listed brand;
(ii) the drug is not on F2.
(3B) This section does not apply in relation to the new brand of the trigger item if:
(a) the new brand of the trigger item is a new presentation of an existing listed brand of a pharmaceutical item; and
(b) the Minister has made a determination under section 99ACBA in relation to the new brand of the trigger item; and
(c) the determination under section 99ACBA has not ceased to have effect.
First new brand price reduction
(4) The Minister:
(a) may, under section 85AD, make a price agreement for the new brand of the trigger item; and
(b) must not make a determination under section 85B in relation to the new brand of the trigger item.
(4A) If, on the day before the determination day:
(a) the approved ex - manufacturer price of a listed brand of the existing item on 1 January 2016; or
(b) if paragraph (a) does not apply--the original approved ex - manufacturer price of the first listed brand of the existing item;
has, by virtue of previous price reductions, been reduced by:
(c) 35% or less, subsection (5) applies; and
(d) more than 35% but less than 60%, subsection (5A) applies.
Note: If previous price reductions have been 60% or more, see paragraph (2)(d).
(4B) If the approved ex - manufacturer price mentioned in paragraph (4A)(a) or (b) is by reference to a different pricing quantity than the pricing quantity on the day before the determination day, the approved ex - manufacturer price mentioned in that paragraph is taken to be the amount that the approved ex - manufacturer price would have been had the pricing quantity been the same as the pricing quantity on the day before the determination day.
(5) Subject to subsections (6) and (6A), the agreed price of the new brand of the trigger item that comes into force on the determination day must not exceed the approved ex - manufacturer price, on the day before the determination day, of the existing brand of the existing item, reduced by 25%.
(5A) Subject to subsections (6) and (6A), the agreed price of the new brand of the trigger item that comes into force on the determination day must not exceed:
(a) 40% of the approved ex - manufacturer price of a listed brand of the existing item on 1 January 2016; or
(b) if paragraph (a) does not apply--40% of the original approved ex - manufacturer price of the first listed brand of the existing item.
(5B) If the approved ex - manufacturer price mentioned in paragraph (5A)(a) or (b) is by reference to a different pricing quantity than the pricing quantity on the day before the determination day, the approved ex - manufacturer price mentioned in that paragraph is taken to be the amount that the approved ex - manufacturer price would have been had the pricing quantity been the same as the pricing quantity on the day before the determination day.
Apportioning if pricing quantity changes
(6) If the pricing quantity of the existing brand of the existing item on the day before the determination day is different from the pricing quantity of the existing brand of the existing item on the determination day, then, for the purposes of subsections (5) and (5A), the approved ex - manufacturer price of the existing brand of the existing item on the day before the determination day is taken to be the amount worked out as follows:
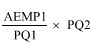
where:
"AEMP1" means the amount that was the approved ex - manufacturer price of the existing brand of the existing item on the day before the determination day.
"PQ1" means the pricing quantity of the existing brand of the existing item on the day before the determination day.
"PQ2" means the pricing quantity of the existing brand of the existing item on the determination day.
Ministerial discretion not to apply, or to reduce, statutory price reduction
(6A) The Minister may, by notifiable instrument, determine that:
(a) the agreed price of the new brand of the trigger item that comes into force on the determination day is to be equal to the approved ex - manufacturer price, on the day before the determination day, of the existing brand of the existing item; or
(b) the agreed price of the new brand of the trigger item that comes into force on the determination day must not exceed the approved ex - manufacturer price, on the day before the determination day, of the existing brand of the existing item, reduced by a lower percentage than would otherwise result from the operation of subsection (5) or (5A) in relation to the determination day.
(6B) If the Minister makes a determination under paragraph (6A)(a), subsections (5) and (5A) are taken not to have applied to the trigger item.
(6C) In making a determination under subsection (6A):
(a) the Minister must take into account what the agreed price of the new brand of the trigger item would otherwise be under this section in relation to the particular determination day if a determination were not made; and
(b) the Minister may take into account any other matter that the Minister considers relevant.
Section does not limit Minister's powers
(7) This section does not limit the Minister's powers, after the determination day, to make:
(a) further price agreements; or
(b) determinations under section 85B;
for the new brand of the trigger item.